
SUMMARY:
-
Microdispensing enables the production of highly uniform microparticles
-
These particles can be used to encapsulate therapeutic agents, offering sustained drug release over several months.
-
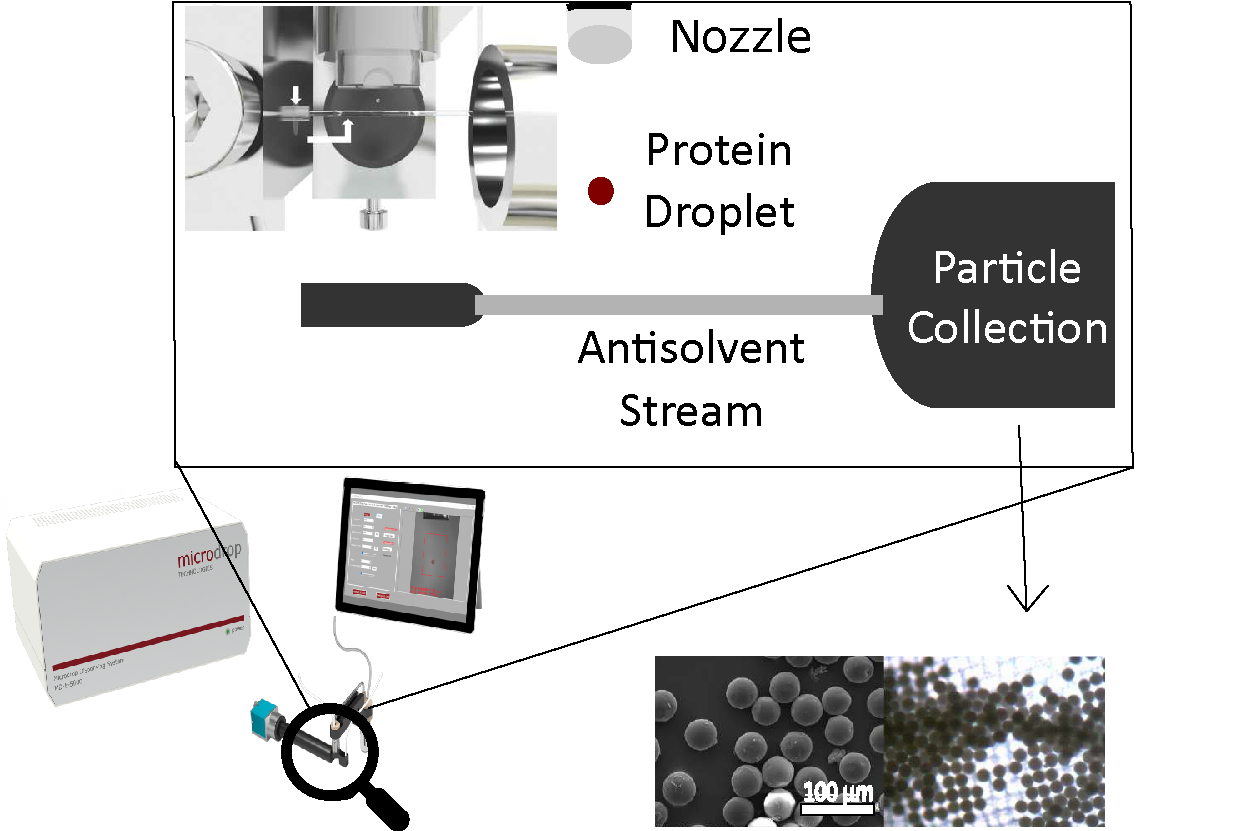
The technique involves drop-on-demand inkjet printing, followed by rapid phase separation triggered by an anti-solvent jet, resulting in particles with narrow size distributions (typically 20–60 μm).
Background
Bioresorbable polymer microparticles are widely used for sustained drug delivery in the treatment of chronic diseases. Precise control over their physical properties enhances reproducibility, therapeutic performance, and manufacturing efficiency. Using a microdrop dispenser head, these microparticles can be produced via rapid phase separation, yielding highly uniform, injectable particles from PLGA and PLA/PLGA blends. This use case is based on the work of Daniel Palmer and colleagues, who generously provided images for this page. Their company Biodexa Pharmaceuticals, which was previously known as Q Chip.
Reasons for Using Inkjet Printing
Microdispensing offers several advantages:
-
Precision Droplet Generation Inkjet technology enables the creation of picolitre-sized droplets with high accuracy, ensuring consistent particle size and morphology.
-
Uniformity: Rapid phase separation results in highly uniform PLGA and PLA/PLGA microparticles, enhancing drug delivery performance.
-
Material Compatibility: Inkjet systems can handle non-conventional “inks,” including polymer solutions with pharmaceutical payloads, without the limitations of emulsion-based methods.
Fabrication Process
Drop-on-demand inkjet printing using the MD-E System was employed to dispense polymer solutions (PLGA or PLA/PLGA dissolved in DMSO). The ejected droplets were immediately exposed to a transverse jet of anti-solvent (e.g., water), inducing rapid phase separation. The resulting microparticles were collected through filtration.
This process yielded highly uniform PLGA and PLA/PLGA microparticles with narrow size distributions, typically ranging from 20 to 60 μm in diameter.
Encapsulation experiments using therapeutic peptides such as ciclosporin A and octreotide demonstrated successful incorporation into the particles. The formulations exhibited sustained release profiles over several months.
Utility and Outlook
Especially given the growing emphasis on personalized medicine in current research, the approach outlined here shows strong potential for high-throughput applications. It has also been demonstrated that the process is scalable: by using inkjet nozzle arrays, production rates exceeding 1 million particles per second can be achieved, supporting industrial-scale manufacturing.
References
We are grateful to Daniel Palmer and colleagues for sharing their work and allowing us to use their images from Biodexa Pharmaceuticals PLC. Their research can be found here https://doi.org/10.1016/j.ces.2017.03.038. Moreover, they hold several patents on the technology which do offer a good background read including: